Among the various non-oxide ceramics that have found commercial applications, silicon carbide (SiC) is the leader. The attractive properties, such as good specific strength and Young’s modulus as a function of the temperature, the specific stiffness, relatively low weight, corrosion and erosion resistance and, most of all, easy availability in complex engineering shapes, have made SiC an attractive alternative to the hard metal compositions.
Silicon carbide is often referred to as “carborundum”: this vernacular term commemorates a word coined by Edward G. Acheson in 1892 to describe crystals that he made in an experiment which had the real goal of making a diamond-like crystal from carbon and alundum. Using a primitive furnace of his own design, he in fact made SiC. Acheson immediately designed a more efficient electric furnace and soon a profitable business with the jewelry trade was established. A century later, the furnaces used to make almost all SiC worldwide follow his original design concept.
Naturally occurring SiC was known (moissanite) and small quantities had previously been made on a laboratory scale but not always recognized; but Acheson quickly recognized the commercial potential of his invention. In the original Acheson’s furnace, two electrodes connect to a graphite core laid within a surrounding mixture of reactant carbon, salt and sand. When an electric current is passed through the graphite core, it heats the surrounding reactants, resulting in the formation of a hollow cylinder of SiC and the expulsion of carbon monoxide (CO) gas. The final SiC cylinder has graphite in its interior and partially reacted charge materials in an annular zone on its periphery.

The chemical reaction for the manufacture of SiC from silica (SiO2) and carbon is written as:
| SiO2(s) + 3C(s) = SiC(s) + CO(g) | (1) |
Beta-SiC is synthesized through the gaseous intermediate SiO(g) with the following reactions being most important:
| SiO2(s) + C(s) ⟶ SiO(g) + CO(g) | (2) |
| SiO2(s) + CO(g) ⟶ SiO(g) + CO2(g) | (3) |
| C(s) + CO2(g) ⟶ 2CO(g) | (4) |
| 2C(s) + SiO(g) ⟶ SiC(s) + CO(g) | (5) |
SiO(g) is initially formed at the contact points of the carbon and the silica, according to the reaction (2). However the high rate of SiO(g) synthesis indicates that the gas/solid mechanism, through the reactions (3) and (4), is the one which render the reaction (5) possible, once the C/SiO2 contact points are consumed. The starting carbon source has a substantial influence on the product SiC morphology and the rate of the reaction, with the product SiC crystallites resembling the starting carbon crystallites prior the grain growth. Then, as expected, SiC crystallites sinter and grow with increasing temperature and reaction time. Although starting SiO2 crystallite size has little effect on the reaction rate, finer carbon crystallites react faster than larger ones. The reduction of SiO(g) with C(s) is rate limiting. All of these reactions characteristics are consistent with a gas-solid model in which reaction of SiO(g) proceeds at the surface of the carbon particles. As the SiC product layer grows, the reaction surface decreases and leads to a decrease in the rate of reaction.
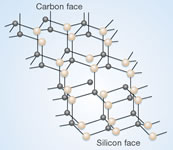
Depending on the synthesis temperature, SiC can be prepared with several crystallographic orientations. Low temperature reaction usually lead to the formation of β-SiC, with a cubic crystal structure. Higher temperature processes, which are more prevalent, form one or more hexagonal modifications. These modifications, which differ only by the position of the atomic layers, are referred to as α-SiC.



The carbides (of tungsten, boron, silicon, titanium and tantalum) have been used for a variety of applications over the years. In general they are very yard and possess excellent wear resistance as well as oxidation resistance. Powder green forming technologies are usually employed in manufacturing engineered products of these materials.
The fabrication of SiC via hot pressing at temperature greater than 2000°C has been practiced for a long time using aluminum and similar elements as hot pressing aids. However, the earliest use of shapes was in the form of a silicon-SiC composite made by the reaction-sintering or reaction-bonding process (RBSC). Typically the SiC grains are mixed with carbon in various forms and the mixture heated in contact with molten silicon. The term reaction sintering arises from the reaction between carbon and silicon to form SiC. The carbon use in the process is converted to new SiC which bonds to the original SiC particles.
The widespread use of SiC for structural applications became a reality when pressure-less densification methodology was discovered in the early 1970s. Both β and α forms could be sintered to very high densities by using boron and carbon additions in various forms. The fact that aluminum and its compounds can also be used for densification was demonstrated in Europe. In the 1980s, more discoveries were made in using transition elements and compounds to help pressureless sintering of SiCs, primarily in Japan.
With respect to the applications, among the greatest successes were the use of SiC in the metallurgical sector – which burgeoned as steel and iron production increased, and the automotive industry grew and expanded. Today those industries utilise almost 60% of global SiC production.
During the 20th Century, SiC continued to evolve to become a critical ingredient in many of the most high-tech, high performance products and applications – from metallurgy to ceramics, abrasives, and electronics, to name a few – with new opportunities emerging as green energy applications become more prevalent.



New opportunities are on the drawing board today. For all of its progress and contributions to energy saving technologies, SiC production remains an energy intensive material to produce. As pressures increase to reduce CO2 emissions, new ways are being sought to reduce environmental impacts while maintaining consistent, high performance materials. Until recently, few options appeared viable: the final goal of TyGRe project perfectly lies within this frame.








